Peri-implantitis is no longer an unavoidable complication. A prevention-based approach to implant dentistry – supported by long-term clinical data – demonstrates how targeted design principles and biological integration can dramatically reduce the incidence of peri-implant diseases.
Soft-tissue bond – the missing element for a dynamic peri-implant defense
For the first time, a biochemical bond between the peri-implant soft tissue and the transmucosal surface of a dental implant was histologically observed and scientifically documented. This newly discovered soft-tissue bond, which is specific to the Patent™ Dental Implant System, plays a critical role in maintaining long-term peri-implant tissue health, thereby ensuring long-lasting esthetic results, by creating a dynamic defense against downward migration of plaque, inflammation and disease progression.
Commenting on the clinical implications of this novel soft-tissue bond, distinguished researcher Dr. Roland Glauser remarked: “In contrast to conventional implants, which often exhibit pocket depths of 5 mm or more, Patent™ Implants show probing depths of no more than 3 mm – comparable to a healthy natural tooth. This finding was impressively confirmed in a 9-year study by the University of Düsseldorf.”
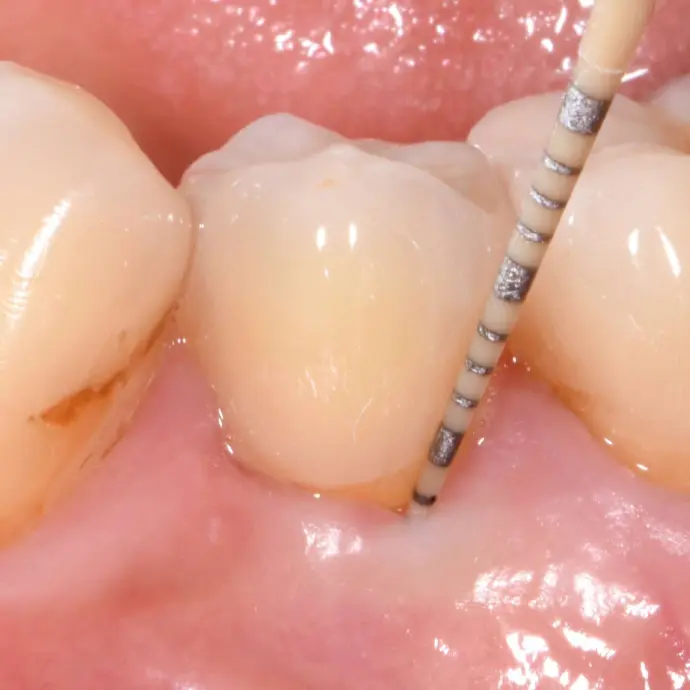
Clinical situation 4.5 years post-op: Integrated Patent™ Implants show probing depths (≤3 mm) comparable to healthy natural teeth (© Dr. Roland Glauser).
Long-term studies conducted at leading European universities have shown that, thanks to the novel soft-tissue bond of the Patent™ Implant, peri-implantitis can be prevented, mucositis minimized, and esthetics maintained over extended periods of function—even in high-risk implant patients with active periodontitis, systemic diseases, poor oral hygiene, or those who smoke.[1, 2]
References:
1. Brunello G, Rauch N, Becker K, Hakimi AR, Schwarz F, Becker J. Two-piece zirconia implants in the posterior mandible and maxilla: A cohort study with a follow-up period of 9 years. Clin Oral Implants Res. 2022;33(12):1233-1244. doi:10.1111/clr.14005
2. Karapataki S, Vegh D, Payer M, Fahrenholz H, Antonoglou GN. Clinical performance of two-piece Zirconia dental implants after 5 and up to 12 years. Int J Oral Maxillofac Implants. 2023;38(6):1105-1114. doi:10.11607/jomi.10284
1. Brunello G, Rauch N, Becker K, Hakimi AR, Schwarz F, Becker J. Two-piece zirconia implants in the posterior mandible and maxilla: A cohort study with a follow-up period of 9 years. Clin Oral Implants Res. 2022;33(12):1233-1244. doi:10.1111/clr.14005
2. Karapataki S, Vegh D, Payer M, Fahrenholz H, Antonoglou GN. Clinical performance of two-piece Zirconia dental implants after 5 and up to 12 years. Int J Oral Maxillofac Implants. 2023;38(6):1105-1114. doi:10.11607/jomi.10284





