Predictable osseointegration of dental implants with a strong soft-tissue seal arguably leads to healthy hard and soft tissue that are maintained in the long term. To scientifically substantiate this view, Swiss-based implantologists and researchers Dr Roland Glauser and Dr Peter Schüpbach recently conducted a study on mini-pigs to evaluate the healing around freshly inserted Patent™ zirconia implants. At the 2021 International Dental Show (IDS) in Cologne, Zircon Medical had the chance to speak with Dr Glauser about the study design and the implications of his findings regarding clinical application.
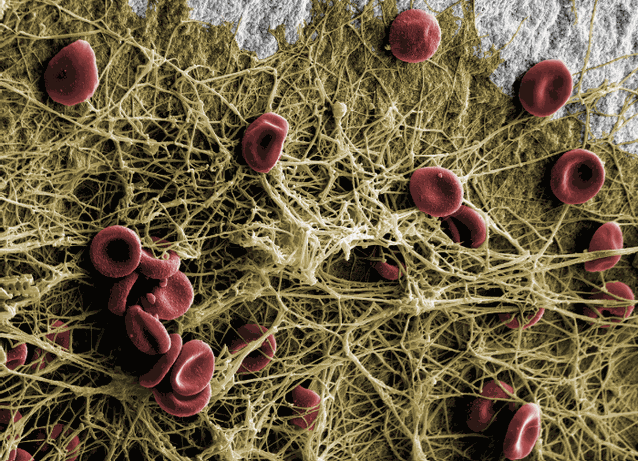
Human blood on the hydrophilic Patent™ surface: Within ten minutes the fibrin network is attached to the implant surface.
This attachment is a prerequisite for contact osteogenesis.
This attachment is a prerequisite for contact osteogenesis.
Dr Glauser, you have been using and investigating zirconia implants for years. What needs to be considered when working with zirconia implants?
As a clinical user, I find it important that long-term data is available for the product I am using and that an implant design has been chosen that respects the characteristics of the implant material. Fast and safe osseointegration is particularly important, as this reduces risks for the patient.
What factors play a primary role when it comes to the healing of hard and soft tissue around dental implants?
First of all, the protocol established for each individual implant system on the market is of great importance. The practitioner must know and accept this in detail and integrate it into his or her clinical workflows. In addition, an awareness of how to correctly and gently handle hard and soft tissue in the context of implant restorations is crucial. Furthermore, the hardware components that interact and react with the surrounding hard and soft tissue immediately after insertion are crucial for a successful long-term treatment outcome. If these three points are taken into account, successful and rapid osseointegration can be achieved.
Two different mechanisms of bone healing around implants have been documented. Distance osteogenesis is the first documented mechanism of bone healing and was first described in the late 1960s. Within the context of distance osteogenesis, which proceeds rather slowly and is primarily found with classically machined surfaces, fresh bone forms in the osteotomy in the direction of the free surface of a placed implant. With modern moderately rough and osteoconductive implant surfaces, we increasingly see the mechanism of contact osteogenesis: starting from a single point of contact with the bone, a migration of bone-forming cells along the free implant surface begins, accelerating the overall process of bone healing around the implant.
Two different mechanisms of bone healing around implants have been documented. Distance osteogenesis is the first documented mechanism of bone healing and was first described in the late 1960s. Within the context of distance osteogenesis, which proceeds rather slowly and is primarily found with classically machined surfaces, fresh bone forms in the osteotomy in the direction of the free surface of a placed implant. With modern moderately rough and osteoconductive implant surfaces, we increasingly see the mechanism of contact osteogenesis: starting from a single point of contact with the bone, a migration of bone-forming cells along the free implant surface begins, accelerating the overall process of bone healing around the implant.
What further properties must an implant possess to achieve such accelerated hard- and soft-tissue healing?
With a view to the current literature, the architecture and the structure of the implant surface as well as its chemical properties are crucial for achieving a rapid healing cascade. In the case of modern implant surfaces and in particular the surface of the Patent™ Dental Implant System, which I have investigated together with Dr Peter Schüpbach as part of an animal model study, another decisive mechanism can be observed: bone debris and a bone smear layer are found along the free implant surface that has not yet been in contact with the surrounding bone. I have been conducting research on this very topic together with Dr Schüpbach for 14 years now, and we have found that this area in particular has high osteogenetic potential. Furthermore, in our research on the Patent™ Dental Implant System, we have found that especially its highly rough surface in the endosseous part, which is at least twice as rough as other well-established implant surfaces, has a beneficial impact on bone healing. With its highly rough surface, the Patent™ Implant is virtually at the other end of the scale of Albrektsson and Wennerberg. Owing to the contact osteogenesis described earlier, which is facilitated by this highly rough implant surface, the Patent™ Implant system achieves an enormously high bone–implant contact already a short time after insertion.
You mentioned an animal model study that you conducted in collaboration with Dr Schüpbach to evaluate the healing around Patent™ Implants. How was this set up, and what were your results?
Since we wanted to create conditions that were as controlled as possible, we chose a preclinical study design. To establish real-life transferability, we decided to work on mini-pig animal models and did what is done in clinical practice in the same way: we extracted teeth and subsequently placed implants in immediate implant placement procedures. After healing times of four and eight weeks, respectively, we removed the placed control and test implants. The control implants were established titanium implants and the test implants were Patent™ zirconia implants. We deliberately avoided influencing factors such as bone augmentation. In cross section, we were then able to take a close look at the healing of the soft tissue and bone at the two explantation time points. For us, the unknown variable was what happens at the free surface at which the implant comes into direct contact with the bone.
Upon evaluating the data, our preliminary conclusion is that the healing success of the Patent™ Implant surface outperforms that of all other surfaces investigated in comparable animal model studies to date. Whereas a bone–implant contact value of just 30–40% has been documented for machined implants after a healing period of 12 weeks—the steady state in mini-pigs—and a value of about 60% at the time of the steady state has been found in studies on modern implant surfaces, Patent™ Implants achieved a value of about 70% already four weeks after insertion.
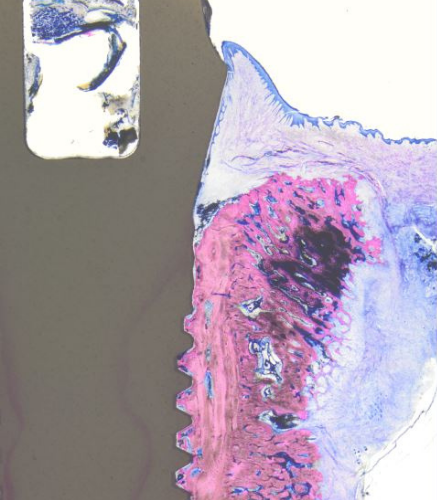
Histology showing hard- and soft-tissue adaptation at four weeks of healing (© Dr Peter Schüpbach).
Furthermore, we found that the steady state for Patent™ Implants was already reached after a healing time of eight weeks at the latest. For everyday clinical practice, this means that regardless of the protocol that is being followed, practitioners and patients have greater security at an earlier stage and they can assume that even 20 years after implant placement—with a possible gingival retraction of 1.0–1.5 mm—no major problems will occur. As far as I am concerned, this is the compelling argument for Patent™ Implants from a biological point of view.
The second surprising finding from our study is that the vertical histoarchitecture of sulcular epithelium, junctional epithelium and connective tissue at the soft-tissue level around Patent™ Implants has a highly favorable structure and that the soft-tissue seal around these implants remained consistently above soft-tissue level after a healing period of four weeks. In contrast, significant accumulation of calculus and plaque was observed around the control implants, spreading downward towards the junctional epithelium, the point at which the human body begins to react. This is consistent with observations from the literature that plaque accumulation around zirconia implants is significantly lower than around titanium implants.
The second surprising finding from our study is that the vertical histoarchitecture of sulcular epithelium, junctional epithelium and connective tissue at the soft-tissue level around Patent™ Implants has a highly favorable structure and that the soft-tissue seal around these implants remained consistently above soft-tissue level after a healing period of four weeks. In contrast, significant accumulation of calculus and plaque was observed around the control implants, spreading downward towards the junctional epithelium, the point at which the human body begins to react. This is consistent with observations from the literature that plaque accumulation around zirconia implants is significantly lower than around titanium implants.
In conclusion, what do you believe are the advantages of the Patent™ Dental Implant System, as opposed to other leading zirconia technologies on the market?
The Patent™ Dental Implant System is purposefully designed to meet the material characteristics of zirconia. I must say that Zircon Medical has truly mastered the complex manufacturing process. The outcome is a unique hydrophilic surface on the intra-bony part. As mentioned, we have seen in preclinical and clinical investigations on the Patent™ Dental Implant System that the implant outshines all previously documented systems (titanium as well as zirconia systems) in terms of bone healing speed. Not least because of the incredibly positive feedback from patients, I am convinced that the Patent™ Dental Implant System will change many things in dental implantology for the better.
First published in issue 1/21 of ceramic implants – international magazine of ceramic implant technology (OEMUS MEDIA AG).





